A University of Melbourne team has developed a way to grow corneal cells in the lab that may eliminate the need for donor corneas and restore eyesight to millions of people worldwide.
Corneal disease is a debilitating eye condition that leads to blindness and currently affects 10 million people worldwide. The only solution currently available is a cornea transplant. But a new treatment developed by researchers at the University of Melbourne and the Centre for Eye Research Australia (CERA) might one day eliminate the need for donor corneas and reduce the risk of post-transplant rejection.
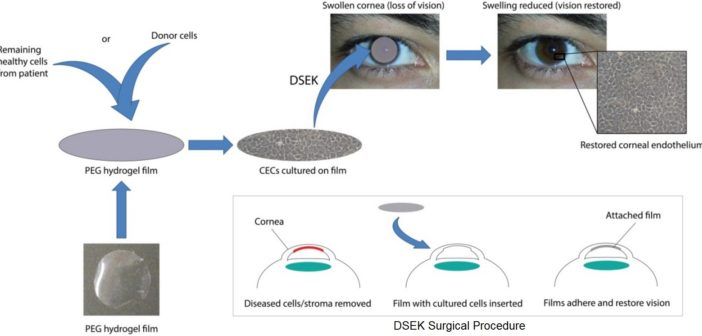
Dr Berkay Ozcelik has developed the world’s first fully synthetic hydrogel film. Transparent and thinner than a human hair, the film can naturally regenerate corneal endothelial cells on its surface. It is implanted via a minute incision in the cornea and dissolves in two months, leaving the new cells behind.
Ozcelik and his co-workers have grown cells on film and successfully implanted them into the eyes of sheep to restore vision. With luck and sufficient funding, they hope to begin human trials in 2018–19, which could lead to the treatment being publicly available by 2022.
“Looking down the microscope and seeing these cells growing on the film for the first time just blew me away,” Ozcelik says. “It was a very rewarding feeling to actually be able to achieve that. “What really drives me is the potential to help millions of people worldwide and give them a much better quality of life.”
The cornea is a very thin, transparent layer at the front of the eye. To stay functional and transparent, it must remain thin – special cells known as corneal endothelial cells aid this by pumping water out of the cornea. But injury, ageing and disease can reduce the number of these cells, causing the cornea to thicken and lose its transparency, eventually leading to blindness.
Corneal transplantation is fraught with difficulties. “It’s difficult to transplant corneas back into patients as the cell layer is very fragile,” says Ozcelik. A patient’s immune system is also primed to recognise and reject this foreign tissue, which means they must take immune-suppressant drugs such as steroids for the rest of their lives.
“About a third of corneal transplants suffer from rejection and have to be replaced,” says Associate Professor Mark Daniell, an ophthalmic surgeon at CERA. Getting donor corneas is also a big problem in much of the world, particularly China, Japan, Russia and India. In China and Japan in particular, there is a cultural aversion to donating corneas, meaning that hundreds of thousands of people remain blind unnecessarily.
A tissue-engineered cornea would overcome the twin problems of a lack of donors and the rejection of corneas. Daniell had been part of a team growing corneal cells in the lab, but he needed a synthetic scaffold that would be implantable in the eye. He approached Greg Qiao, a professor of macromolecular chemistry and engineering at the University of Melbourne, and his then PhD student Ozcelik.
“Berkay developed a whole lot of potential substrates to grow the cells on and to use as a scaffold,” Daniell says. “He came up with this final formulation which is incredibly useful for us. We have something the cells like to grow on and that we can use during the surgery.”